When I started this year of learning chemistry, I knew today was going to be a big day. I had no idea where I would be, or what I would be doing, but I knew today was cause for a celebration. Today, October 23rd, is Mole Day!
If you are not a big chemistry person, you might not know that this is the official day of chemistry. Why October 23rd? To explain, we will have to get into a bit of math.
Right now there are twelve apples in my kitchen. Instead of always referring to that many as twelve, in English I could also call that amount a dozen. The total weight of all of them together is a few pounds.
What if we scaled that up a bit...how many apples are in the entire world? How much do all of them weigh? While this is ridiculous for our everyday calculations, chemists have to deal with these types of numbers all the time!
This is a super simplistic summary, but basically this line of questioning was asked:
-What is the most common element we deal with? Carbon. (Its so common that intro level chemistry is divided based off of it: organic deals with any chemical with carbon, inorganic is any chemical without).
-What is the most common type of carbon? Carbon 12. (There are a few different types of carbon, just like apples can come in all shapes and sizes. 12 is the average size/weight and most common).
-How many Carbon 12 atoms are in 12 grams? 6.022x10^23
That number is so large, that it becomes easier to write it as a math equation rather than as just one number (that number being 602,200,000,000,00,000,000,000). To name it chemists took an abbreviation for "molecule" from German, coming up with mole. So just as a dozen is twelve of something, a mole is 6.022x10^23 of something.
October 23rd was picked since a mole involves 10^23 (June 2nd and 22nd were also in the running, but since most schools are doing finals then October was picked as a better time to celebrate and not be stressed). So today around the nation students have been celebrating by eating chemistry themed snacks, making presentations for younger students, and just having fun with chemistry. I remember fondly my senior year at Hampton where we got to make fire balls in mid air for 9th graders.
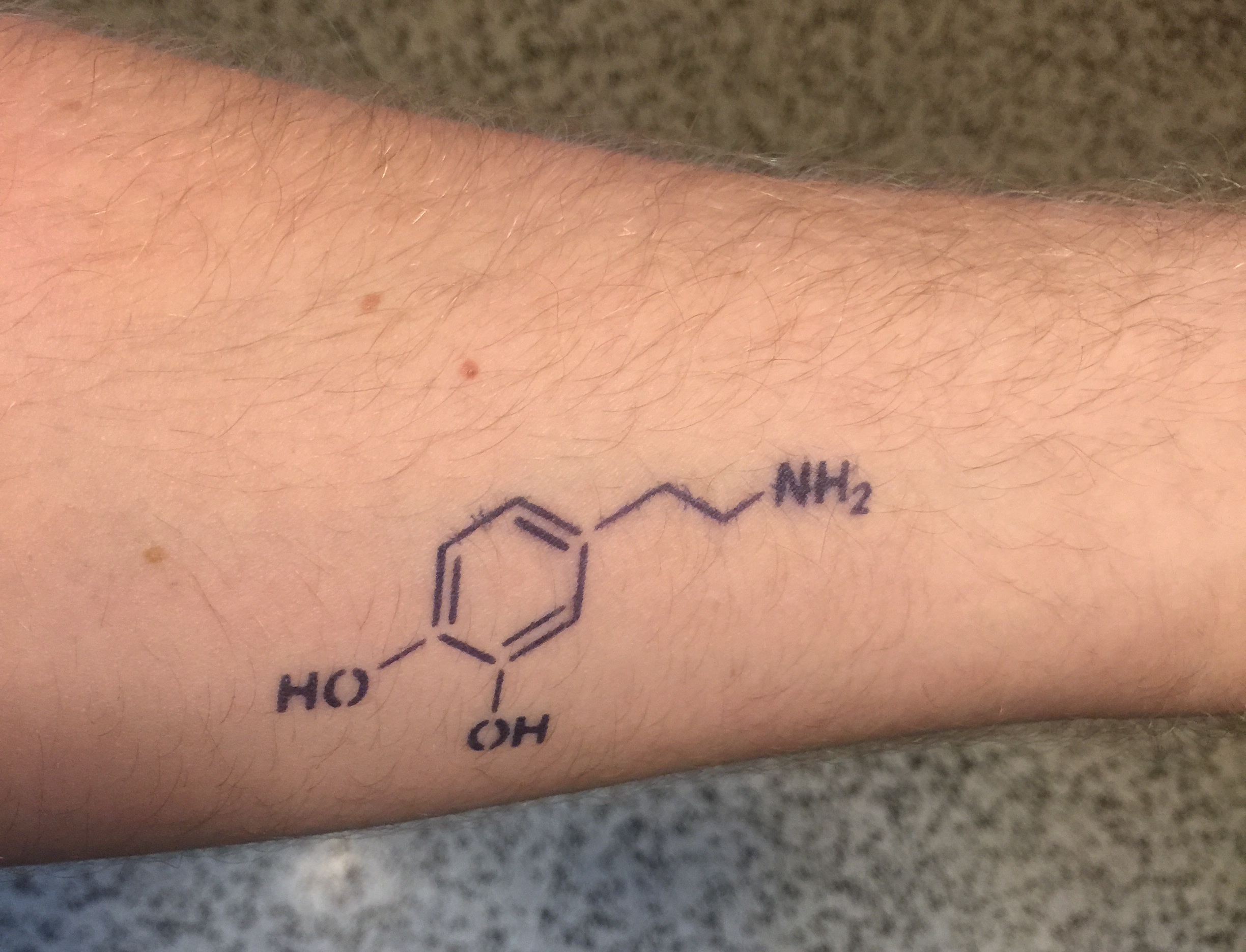
Since I am no longer in school, I knew I couldn't go all out in celebrating this day as I had before. I decided then to get a tattoo for Mole Day! And what would be the perfect way to celebrate? Why the chemical structure of dopamine of course!
Alright fine. I didn't get a real tattoo of dopamine. This is a semi-permanent tattoo that lasts up to two weeks. But the entire process of getting and applying this to my arm released the chemical in a few stages.
Dopamine is responsible for so many things in our bodies. Located between synapses in the brain, this chemical is a large part of the reward system. Look at yourself in the mirror after exercising and are proud of yourself? Dopamine was released. Eat a delicious cake with just the perfect amount of sugar and chocolate? Dopamine was released. Finally turn in that big project you have been working on for months? A huge amount of dopamine was released.
So when I ordered this product online, I got a shot of dopamine in anticipation for a package. When it arrived, I got another hit because of the excitement of a new prize. Seeing it for the first time really clearly on my arm also released some. But as I look at my arm today, I get less dopamine than when I first applied it. I'm still excited by it, but not nearly as much.
Some addictions are the cause of this chemical. It might not be the activity itself, like gambling, that forces you to stay but the possibility of a bigger hit, a bigger high off of dopamine, that makes people keep going back. Conversely if someone's dopamine levels are too low it can result in depression as nothing will be a big enough reward. When it comes to dopamine, and so many other chemicals; moderation is best.
So on this Mole Day go and celebrate chemistry however you see fit! Without all of the chemicals in the known universe working in the symphony they do, we would not have this wonderful life as we know it on earth.